HSP Application note #64
Poly(N-Vinyl-2-pyrrolidone) and Hansen Solubility Parameters (HSP)2010.12.1
HSPiP Team Senior Developer, Dr. Hiroshi Yamamoto
I got very interesting paper.
Solubility Parameters of Cross-Linked Poly(N-Vinyl-2-pyrrolidone-co-crotonic Acid) Copolymers Prepared by g-Ray-Induced Polymerization Technique
Tuncer C¸ aykara
JOURNAL OF MACROMOLECULAR SCIENCE Part A—Pure and Applied Chemistry Vol. A41, No. 8, pp. 971–979, 2004
N-Vinyl-2-pyrrolidone (VP) is known to be a hydrophilic monomer. Because of monomer’s hydrophilic characteristics, PVP is hydrophilic materials, which generally possess excellent biocompatibility with living tissues and extremely low cytotoxicity and used as bio-adhesives.
In the paper, they made several co-polymers and check swelling properties with several solvents.
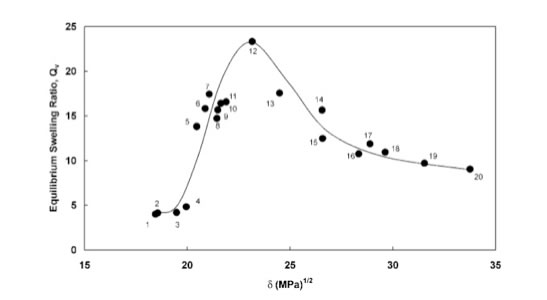
I show the original PVP swelling-Hildebrand SP value plot below.
Solvent No.12 is n-Butanol.
They made 4 co-polymer with Crotonic Acid.
The percentage of Crotonic Acid is different, but the peak of swelling is same.
I re-calculated this swelling properties with Hansen Solubility Parameters(HSP).
Hansen Solubility Parameters (HSP)Hansen Solubility Parameters(HSP) were developed by Charles M. Hansen as a way of predicting if one material will dissolve in another and form a solution. They are based on the idea that "like dissolves like" where one molecule is defined as being 'like' another if it bonds to itself in a similar way.
What can perhaps be surprising is that one can assign HSP to so many different things. Gases like carbon dioxide, solids like carbon-60, sugar, and biological materials like human skin, depot fat, DNA, and even some proteins all have HSP. The list can be continued with drugs, polymers, plasticizers, and in fact any organic material and even many inorganic materials like salts. The only requirement for an experimental confirmation is that the material must behave differently in a sufficient number of test solvents upon contact. Pirika JAVA Demo Applet calculate HSP. HSPLight is available here. |
At first, I need make table like below.
The value is swelling ratios for each PVP polymer and co-polymers.
| No | Name | dD | dP | dH | PVP | CrA4.6 | CrA6.9 | CrA10.1 | CrA13 |
| 1 | Benzene | 18.4 | 0 | 2 | 4.025 | 1.036 | 1.039 | 1.133 | 1.333 |
| 2 | Ethylacetate | 15.8 | 5.3 | 7.2 | 4.208 | 1.036 | 1.039 | 1.234 | 1.047 |
| 3 | Tetrahydrofuran | 16.8 | 5.7 | 5.7 | 4.211 | 1.386 | 1.144 | 1.538 | 1.511 |
| - | |||||||||
| - | |||||||||
| - | |||||||||
| - | |||||||||
| 12 | 1-Butanol | 16 | 5.7 | 15.8 | 23.442 | 17.008 | 10.991 | 9.447 | 8.93 |
| 13 | 1-Propanol | 16 | 6.8 | 17.4 | 17.613 | 15.187 | 8.445 | 7.451 | 7.249 |
| 14 | Ethanol | 15.8 | 8.8 | 19.4 | 15.702 | 8.111 | 6.745 | 6.557 | 6.459 |
| 15 | Dimethyl sulfoxide | 18.4 | 16.4 | 10.2 | 12.516 | 10.213 | 7.221 | 7.156 | 7.742 |
| 16 | Pyrrolidone | 18.2 | 12 | 9 | 10.881 | 9.372 | 7.586 | 7.658 | 6.775 |
| 17 | 1,3-Buthandiol | 16.5 | 8.1 | 20.9 | 11.974 | 8.462 | 7.901 | 7.011 | 7.414 |
| 18 | Methanol | 14.7 | 12.3 | 22.3 | 10.974 | 10.143 | 8.532 | 7.859 | 7.448 |
| 19 | Ethanolamine | 16.8 | 6.8 | 20 | 9.795 | 9.863 | 7.681 | 7.763 | 6.873 |
| 20 | Glycerine | 17.4 | 11.3 | 27.2 | 9.163 | 11.684 | 8.57 | 8.264 | 8.004 |
If the ratio of Crotonic Acid increase, swelling percentage decrease.
But it is very hard to understand this phenomena with Hildebrand SP because the top peaks of all the polymer are same.
I applied Quantitative Sphere algorithm for this problem.
Quantitative Sphere search the best HSP of solute that make largest correlation factor in HSPiP ver.3.1.X.
HSPiP(Hansen Solubility Parameters in Practice)The first edition of HSPiP that appeared in November, 2008, greatly enhanced the usefulness of the Hansen solubility parameters (HSP). The HSP values of over 1200++ chemicals and 500 polymers are provided in convenient electronic format and have been revised and updated using the latest data sources in the second edition (March, 2009). A third edition of the HSPiP appeared in March, 2010. There are now 10,000 compounds in the HSP file which also includes data on density, melting point, boiling point, critical parameters, Antoine constants and much more. The user is able to carry out many different sorts of optimisations of solubility, evaporation, diffusion, adhesion, create their own datasets (automatically if required) and explore the huge range of applications for HSP in coatings, paints, nanoparticles, cosmetics, pharma, organic photovoltaics and much more. The 3rd Edition v3.1 was released on 12 December 2010. Current users can upgrade free (now v3.1.09) by downloading the latest .msi installer from http://hansen-solubility.com The 4th Edition v4.0.x was released on 2 Jan. 2013. The Current users can upgrade with free charge. 2013.1.28 The Visual How to manual of HSPiP. You can understand what HSPiP can do. |
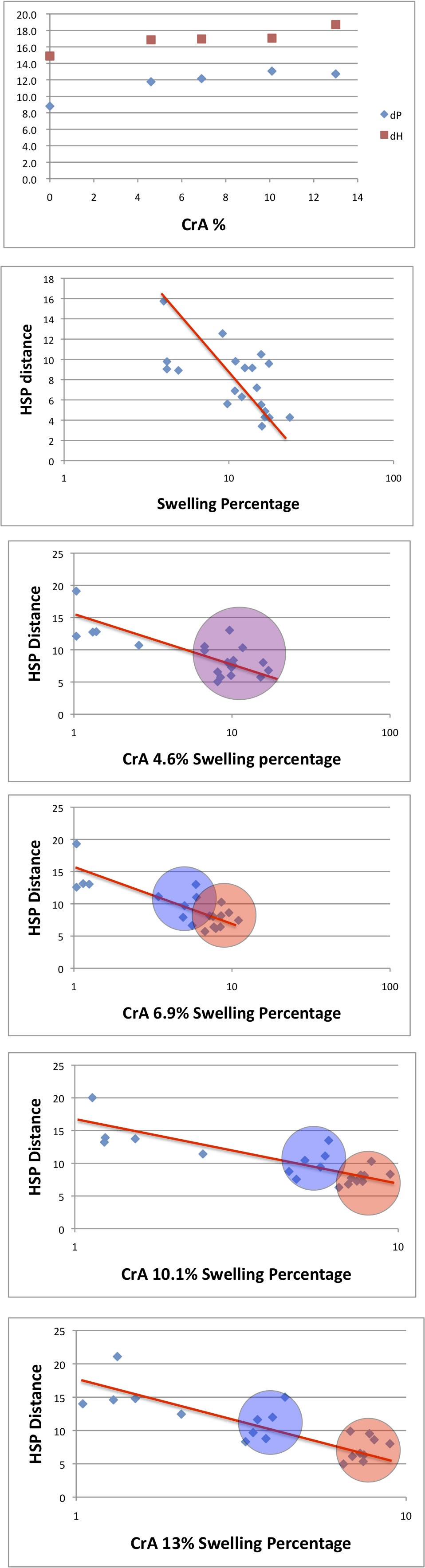
For PVP-100% polymer, Q-Sphere determined the HSP as [17.4, 8.8, 14.9].
| Polymer | Crotonic Acid | dD | dP | dH |
| PVP100% | 0 | 17.4 | 8.8 | 14.9 |
| PVP-CrA4.6 | 4.6 | 17.4 | 11.8 | 16.9 |
| PVP-CrA6.9 | 6.9 | 17.8 | 12.1 | 17.0 |
| PVP-CrA10.1 | 10.1 | 17.8 | 13.1 | 17.1 |
| PVP-CrA13 | 13 | 17.3 | 12.7 | 18.7 |
If the Crotonic Acid percentage increase, the co-polymer dP and dH increase.
So, HSP result are quite different from Hildebrand result.
The swelling percentage are decrease according to increase Crotonic Acid percentage.
But the effect is not identical for solvents.
For average HSP of blue zone solvents are [17.4, 7.3, 10.8]. And these solvents loose swelling power more quickly than red zone solvents [16.6, 9.8, 18.0].
This means Crotonic Acid make hydrogen bond network and larger dH solvents can cut this Hydrogen bonding network.
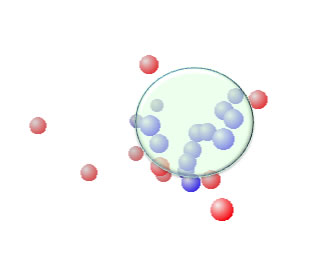
I run classic Sphere program for CrA 13% co-polymer.
The result is [17, 15.8,19.1] Radius=11.65. There are no wrong in/out. But radius is very large.
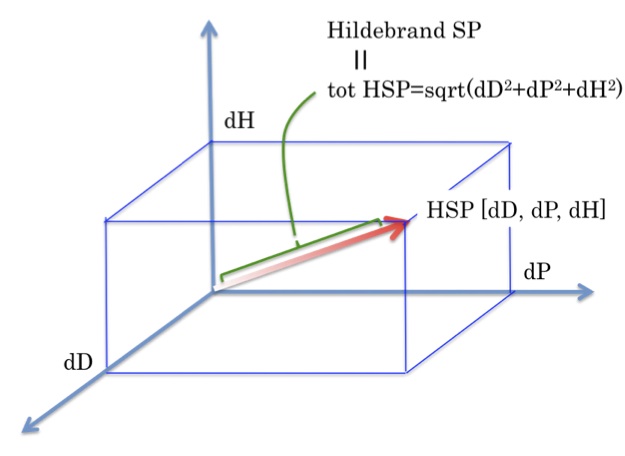
Hansen SphereTo determine if the parameters of two molecules (usually a solvent and a polymer) are within range a value called interaction radius (R0) is given to the substance being dissolved. This value determines the radius of the sphere in Hansen space and its center is the three Hansen parameters.
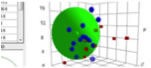
From version 3.1.X, Double Spheres function is available. Pirika provide JAVA 3D Demo Applet to browse the Sphere(s). |
Drag=Rotate, Drag+Shift=Larger/Smaller, Drag+Alt or Command(Window key)=Translate. PVP
CrA4.6%
CrA6.9%
CrA10.1%
CrA13%
If you are using HTML5 enable browser such as Chrome, Safari or FireFox (IE9 is out of support), you will see the Canvas. If you pick solvent, solvent name will appear.
I marked Red if the swelling larger than 7%. The more increasing Crotonic Acid, the less red Spheres.
When I run the Double Spheres program,
[17, 12.2, 20] Radius 8.84 (Typical Alcohol region)
[19.4, 14.9, 5.5] Radius 5.47 (Aldehyde, Anhydride ??)
So, the hydrogen bonding of Crotonic Acid reduce dH value to something like anhydride.
Drag=Rotate, Drag+Shift=Larger/Smaller, Drag+Alt or Command(Window key)=Translate.
If you are using HTML5 enable browser such as Chrome, Safari or FireFox (IE9 is out of support), you will see the Canvas. If you pick solvent, solvent name will appear.