Chemical Engineering: Analytical Solution of Groups
2011.4.14
Lecture note of Dr. Hiroshi Yamamoto
Vapor liquid Equilibrium estimation by ASOG
ASOG stand for Analytical Solution of Groups. ASOG estimate activity coefficients from functional groups. Then estimate Vapor Liquid Equilibrium (VLE) with antoine parameters. Maybe UNIFAC method is much famous.
There are 2 programs that calculate VLE by ASOG.
HTML5 version, if you are using adequate browaser, please try this version.
This program was made with HTML5+CSS+JavaScript. So you need Web Browser that can run HTML5. For PC/Mac Chrome, Safari or FireFox is recommended. iPad with Mobile Safari is also one choice. IE9 may not work because HTML5 implementation is very poor. IE below 9 are not support HTML5.
JAVA version, Mac/PC, with JRE(Java Runtime Environment)
If your browser is not adequate, please use JAVA version.
Here, I will explaine about HTML5 version.
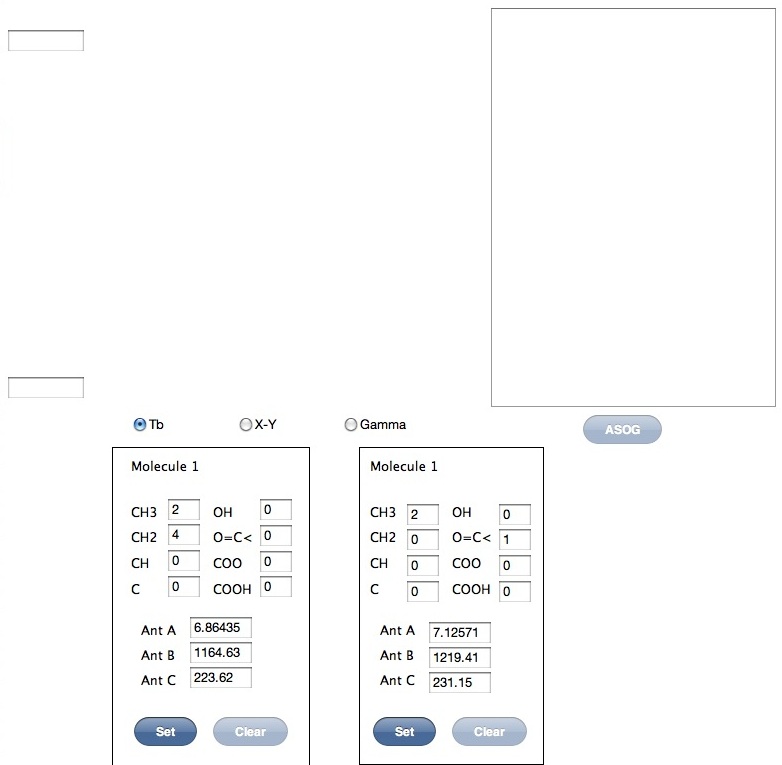
At first, ASOG button is obscure. This program initial molecule 1 is hexane. And second molecule is Acetone. If you set functional groups and Antoine pararmeters then push Set button. If you set both molecules, then ASOG button become active.
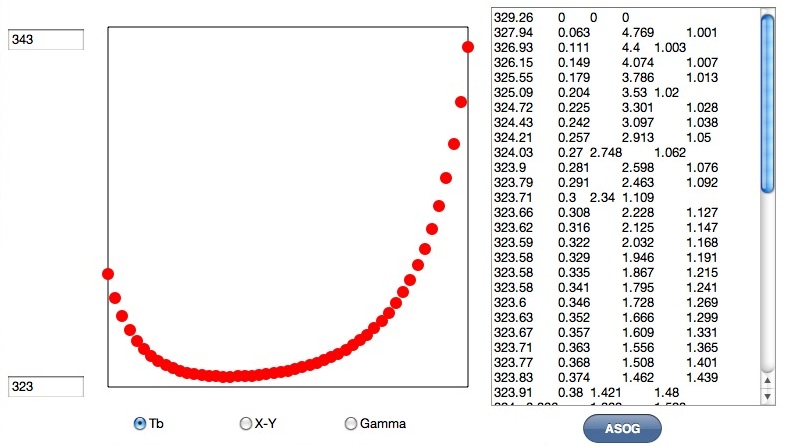
You will get calculation result both graphical data and text data. You can copy the result and paste it to spread sheet for further analysis. First column is boiling Point, Second column is first component vapor ratio, 3rd and 4th column is activity coefficients. The ratio of 2 compornent are divided into 50. Acetone and Hexane make azeotrope composition and boiling point become minimum.
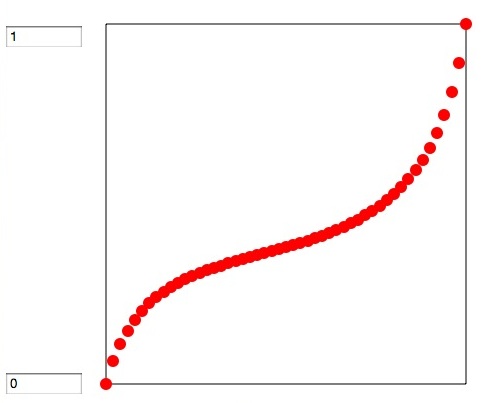
If you select X-Y chart (X is liquid composition and Y is vapor composition) you will get next chart. if liquid and vapor composition is equal, then line bcome Y=X. But with this result, the left side (X1=0, X2=1) Acetone is rich in liquid phase. Around there, in vapor phase hexane is very rich. On the contrary around right side Acetone is rich in vapor phase. The position this curve cross Y=X line, called Azeotrope point.
If you select Gamma, activity coefficients are displayed.
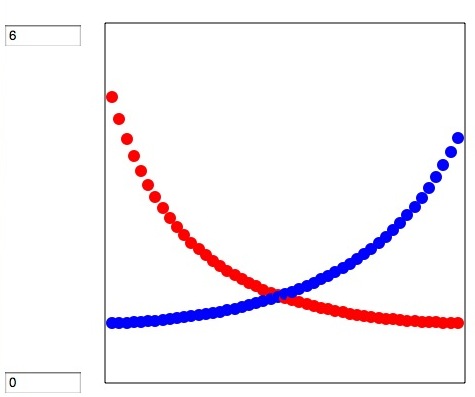
ASOG method is very convenient method to get activity ccoefficients and if you have Antoine parameters for each molecule, you can calculate VLE.
I also developed Antoine parameter estimation method from only molecular structure.
JAVA version Pirika-light
How to use pirika-light
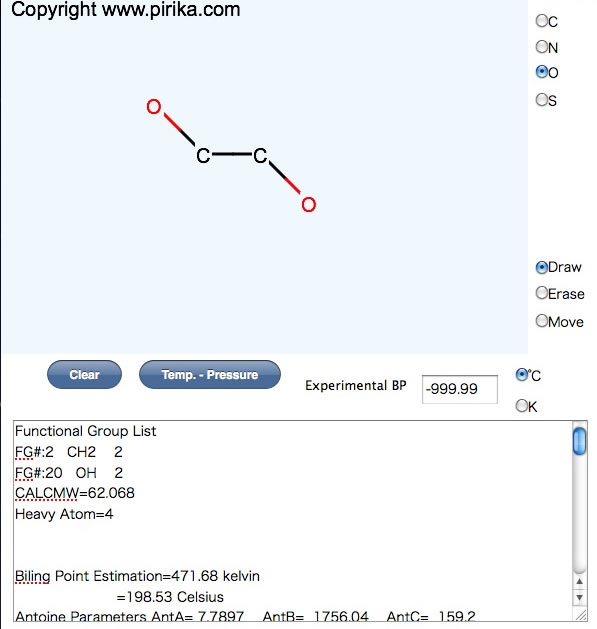
Unfortunately, Antoine parameters are so depend on database. And determining Antoine parameters is not so easy. Please read this article more detail.