Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 21, Cleaning by numbers (HSP for Surfactants)
This
chapter is co-authored by Dr Richard Valpey III of SC Johnson. We are grateful
to Richard for his expert technical input and to his company, SC Johnson, for
giving us permission to use their surfactant HSP data. Responsibility for
errors in this chapter remains with Abbott as per his guarantee.
The good thing about surfactants is that
there are so many to choose from. The bad thing about them is the same –
that there are so many to choose from. Many would-be users of surfactants
despair at having to sort through so many different surfactants in search for
the perfect one.
The search is helped somewhat by well-known
numbers attached to surfactants such as HLB (Hydrophilic- Lipophilic Balance),
Aniline point, KB (Kauri Butanol) value. But these provide surprisingly little
scientific insight into a specific surfactant.
HLB was originally determined by a time
consuming determination of emulsion stability. Griffin measured the stability
of two types of emulsions (oil-in-water and oil-out) formed by a series of oils
in the presence of surfactants. He then fit the results to a systematic ranking
and called it the hydrophile lipophyle balance (HLB). It is time consuming
because approximately 75 emulsions were made for each HLB determination.
Becher suggested that HLB relates to free
energy according to the following equation
![]()
Where: ![]() and
and ![]() are the
free energy of micellisation associated with the lipophilic and hydrophilic
moieties. C1 and C2 are scaling factors.
are the
free energy of micellisation associated with the lipophilic and hydrophilic
moieties. C1 and C2 are scaling factors.
In its original form, HLB was a relative
effectivity index, ranging from 0 to 40. Griffin acknowledged its limitation to
nonionic surfactants.
Davies proposed eliminating this limitation
by computing HLB based on the structure of the surfactant by assigning group
numbers (GN) to various moieties according to the following equation:
![]()
The Davies method, which finds use in
emulsion technology, produces negative HLB numbers, particularly when the
lipophilic contribution is sufficiently large.
Despite difficulties in handling negative
numbers and poor correlation to ionic surfactants, HLB is the most widely used
tool for selecting surfactants.
In 1978, Little suggested a tool that
overcomes these two difficulties. He proposed the following relationship
between the Hildebrand Solubility Parameter δ and HLB. This method which was
originally tested with nonionic and anionic surfactants, correlates poorly with
cationic surfactants.
![]()
Given that HLB themselves frequently offer
little insight to specific problems, and given that we know the limitations of
the Hildebrand parameter, this correlation is not of much help.
Surely it makes sense to provide users with
chemical insights into the functionality of the surfactants via HSP. There has
been remarkably little work on this approach but by combining the earlier work
of Beerbower with the recent work of Valpey we can make some progress.
The key fact is that we can think of
surfactants as having 3 sets of HSP. The first is the hydrophobic portion. The
second is the hydrophilic portion. And the third is the (weighted) average of
the two just as with any mixture of solvents. The last is particularly
important even if you don’t use it directly. Because it is a weighted average, it provides some of
the insights from an HLB. So important is this weighting that we’ve added it to
the software so it’s easy to do.
Here is a list of surfactant HSP partitioned
in the above manner:
|
Surfactant |
δD |
δP |
δH |
|
SLES hydrophobe |
16.0 |
0 |
0 |
|
SLES hydrophile |
20.0 |
20.0 |
20.0 |
|
SLES Average |
16.7 |
8.1 |
8.1 |
|
APG hydrophobe |
15.5 |
0 |
0 |
|
APG hydropile |
23.4 |
18.4 |
20.8 |
|
APG average |
18.8 |
7.6 |
8.6 |
|
Span 80 hydrophobe |
16.1 |
3.8 |
3.7 |
|
Span 80
hydrophile |
18.1 |
12.0 |
34.0 |
|
Span 80 average |
16.1 |
6.1 |
13.2 |
|
Alkyl
sulfosuccinate hydrophobe |
16.0 |
0 |
0 |
|
Alkyl
sulfosuccinate hydrophile |
20.0 |
17.0 |
9.7 |
|
Alkyl sulfosuccinate average |
19.2 |
3.4 |
1.9 |
|
ST-15 hydrophobe |
16.4 |
0.0 |
0.0 |
|
ST-15 hydrophile |
16.2 |
7.8 |
10.4 |
|
ST-15 average |
16.3 |
3.6 |
4.8 |
Table 1‑1 Estimated values for the three characteristic sets of HSP for
typical surfactants. There is not yet experimental data to verify these estimates.
Let’s look more closely at Span 80 –
Sorbitan oleate.
The “oleate” part can be imagined as methyl
oleate with HSP of [16.2, 3.8, 4.5], or simply as [16, 0, 0] representing the
pure hydrocarbon chain. We’ve chosen a group contribution method that gives the
values shown below. The sorbitan can be calculated as [19, 13, 21] with a molar
volume ~ 100.
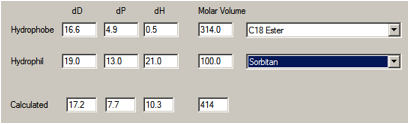
Figure 1‑1
The weighted average (calculated by summing
the individual energies then dividing by the combined molar volume) is
therefore biased towards the hydrophobic end – giving [17.2, 7.7, 10.3].
If Span 20 were considered, sorbitan monolaurate, then the individual HSP don’t
change much, but the reduced molar volume of the laurate moiety (~215) shifts
the average to [17.5, 9.2, 12.1].
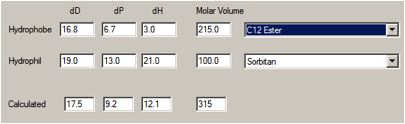
Figure 1‑2
No doubt you’re starting to see the
problems with this approach. There are a few assumptions that have to be made.
Where do you draw the line between hydrophobe and hydrophile? How do you
estimate the HSP and molar volumes for the chunks into which you’ve divided the
molecule?
We’ve attempted to answer some of those
questions for you by providing our best estimates of many of the common groups
used in surfactants. By selecting one of the hydrophobes and one of the
hydrophiles (it’s up to you whether that combination can actually exist) you at
least have a reasonable starting point for your own explorations. But our
values are only for guidance, you should use your own judgement for your
particular surfactants.
For the 3rd Edition we’ve added
an optional Y-MB calculation of the surfactant HSP. Wherever there are
meaningful SMILES values for the head and tail and also meaningful Y-MB
fragments available, the head and tails SMILES are stuck together into a single
SMILES and sent to Y-MB.
At this stage in surfactant research we
have no good data to give you. Instead we’ll go out on a limb and make some
predictions. Let’s take 5 standard “soils” (see the Handbook for an explanation of these 5. The HSP numbers in the Handbook differ from those shown here)
|
No. |
Soil |
δD |
δP |
δH |
|
1 |
ASTM Fuel “A” |
14.3 |
0 |
0 |
|
2 |
Butyl Stearate |
14.5 |
3.7 |
3.5 |
|
3 |
Castor Oil |
15 |
6 |
8 |
|
4 |
Ethyl cinnamate |
18.4 |
8.2 |
4.1 |
|
5 |
Tricresyl
phosphate |
19 |
12.3 |
4.5 |
Now let’s calculate the distance between
each of these soils and 5 surfactants
|
Surfactant |
1 |
2 |
3 |
4 |
5 |
|
SLES |
14.5 |
10.6 |
8.4 |
2.8 |
2.6 |
|
APG |
14.6 |
10.7 |
7.8 |
4.6 |
6.2 |
|
Span 80 |
15.0 |
10.5 |
5.6 |
10.4 |
12.2 |
|
Alkyl sulfosuccinate |
10.5 |
9.5 |
10.7 |
5.5 |
9.3 |
|
ST-15 |
7.2 |
3.8 |
4.8 |
6.3 |
10.2 |
If you believe this approach to
surfactants, then from the table you can instantly work out that each stain has
an optimal surfactant. Stain 1 would best be removed by ST-15, though the
distance is so large that it might not work at all. ST-15 will be also be best
for stains 2 and 3, with more success, and SLES would be best for stains 4 and
5.
The “if” at the start of the previous
paragraph is rather important. Classic thinking about surfactants tends to
assume that the hydrophobic tail does the interaction with the soil and the
hydrophilic head does the interaction with the water so that the tail + soil
get swept away. There is an obvious problem with this classic thinking. The
tails of most surfactants are remarkably similar and therefore the cleansing power
should be fairly similar as long as the head is swept away in the water. The
very large differences in cleaning power of different surfactants are therefore
not naturally explicable using such simple ideas. The HSP model suggests an
alternative approach to rational removal of soils.
Of course, the classic model includes the
formation of micelles as the actual cleaning agents, and the different chains
give different critical micelle concentrations and, therefore, different
behaviour in the cleaning environment. Notions such as Critical Packing
Parameter depend strongly on the relative size of head and tail. The simplistic
HSP approach says nothing about this important element of surfactant behaviour.
But of course the different chain lengths will also have different HSP and
molar volumes, which, in turn, determine their solubility in water and, even
more importantly, their relative solubility in the two phases and thus the
delicate balance which appears as the PIT – Phase Inversion Temperature.
Perhaps the most interesting aspects of HSP and surfactants will be their use
in non-aqueous dispersions, where matching HSP of the surfactant ends to the
HSP of the respective phases would seem to be a helpful exercise.
Nevertheless, we’re happy to predict that
an intelligent use of HSP will prove highly insightful for many cleaning
applications. We have some evidence from our own commercial activities that
these predictions are indeed helpful. But despite the fact that this approach
was first suggested by Beerbower many years ago, only recently has it been
looked at with fresh eyes and more powerful ways of predicting HSP values. The
Inverse Gas Chromatography (IGC) technique discussed in the Chromatography
chapter gives hope that the HSP of numerous surfactants will be measured
experimentally, which will be an important addition to our knowledge base. We
are confident that we will be hearing more about coming clean with HSP.
Update
for the 4th Edition
The big advance in surfactant theory has
come from the HLD theory of Salager as extended by Acosta to form HLD-NAC. This
simple, numerical approach offers great power and puts to shame naïve
ideas of HLB and invalidates many of the formulation ideas behind CPP (Critical
Packing Parameters). This eBook is not the place to discuss HLD-NAC. The free
software and apps provided at www.stevenabbott.co.uk/HLD-NAC
gives formulators a quick way to learn how to apply the theory.
But HLD-NAC requires knowledge of the “oil”
with which one is formulating. In particular it requires the Equivalent Alkane
Carbon Number (EACN) for the oil. HSPiP can now estimate the EACN from a SMILES
input. The estimation is only as good as the dataset used to model it. There is
an unfortunate lack of reliable EACN values across a wide range of molecules.
As HLD-NAC becomes more used and EACN values for more oils are measured the
estimation scheme will become steadily more reliable.
E-Book contents | HSP User's Forum