Hansen Solubility Parameters Abnormality of methanol
2013.8.4
HSPiP Team Senior Developer, Dr. Hiroshi Yamamoto
When we discussed about Methanol animosity at DC IV, Charles gave us very important information. That was came from very old Book, “Macromolecular Solutions: Solvent Property Relationships in Polymer Pergamon Pr (1982/03)”, Raymond Benedict Seymour (Author) . It is very hard to obtain that book now, so I introduce you the abstract briefly.
P166-
Elastomer/Gasoline Blends interaction I.
Effects of Methanol/Gasoline Mixtures on Elastomers.
In this chapter, they examined the fluorocarbon elastomer’s swelling ratio to various alcohols. Ethanol swells elastomer just 5.4% (volume gain) but Methanol swells 100%!
And the author’s conclusion was that (Page 186).
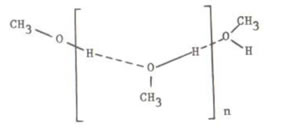
“Comparing Methanol’s HSP to elastomer’s HSP, the large difference is in the value of δH. This led us to believe that methanol in the pure form exists in a highly hydrogen bonded structure and hence has a lowerδH value.” And they suggested next structure as Methanol cluster.
Let’s examine this phenomenon more closely.
Trouton’s rule: states that the entropy of vaporization is almost the same value, about 85–88 J K−1 mol−1, for various kinds of liquids.[1] The entropy of vaporization is defined as the ratio between the enthalpy of vaporization and the boiling temperature. (From WikiPedia)
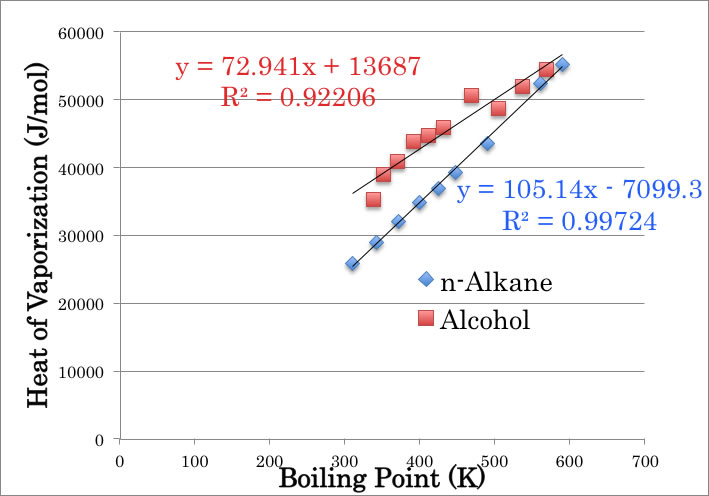
So, if I plot boiling point to heat of vaporization @ BP, I can find very good correlations. Left red square (Methanol) is a little bit out of the line, but not so bad.
I don’t know whether this rule is known to public, (so temporally I called this rule “Pirika’s rule”), but if I plot Molecular Volume (calculated from liquid density) to Heat of vaporization at 25℃, I can get much beautiful correlation.
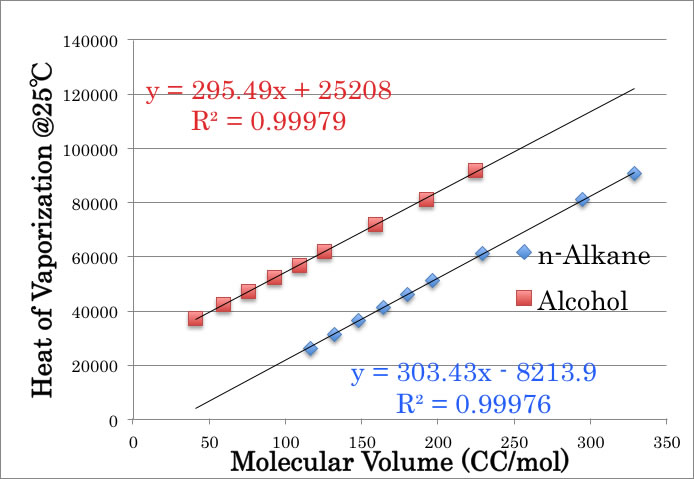
This Pirika’s Rule is much important than Trouton’s rule in the field of Hansen Solubility Parameters (HSP). Because in HSP, we use the following equation to calculate tot HSP:
Tot HSP= SQRT( (Hv@25 – RT)/Mol Volume)
So, Hv@25 and Mol volume are very important. From this Pirika’s rule, I can’t find any abnormality with Methanol.
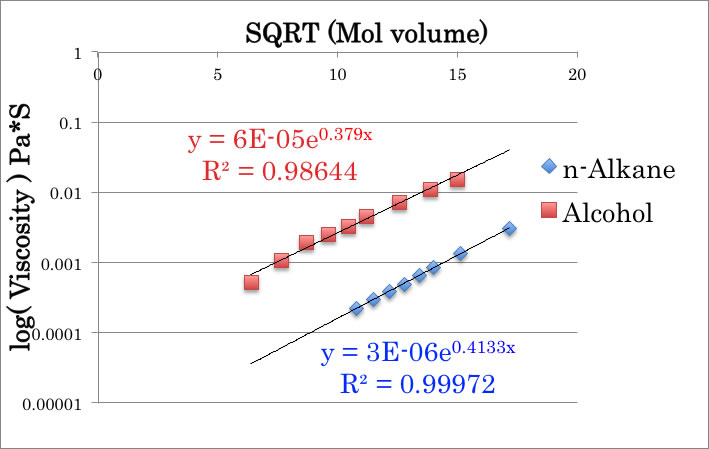
If only methanol make linear hydrogen bonding cluster and Ethanol or other alcohol don’t make cluster, some other observable thermo-dynamic properties should change. For example, longer hydrogen bonding cluster’s viscosity will become different from other liquids.
Again, I don’t know whether this rule is known to public or not, but as “Pirika’s rule 2”, square root Mol volume to log viscosity has linear correlation. The Methanol behavior is very normal.
Actually if I apply Methanol HSP [14.7, 12.3, 22.3] to solubility of n-eicosane case, I can believe Methanol’s HSPs are correct. The n-eicosane will not dissolve because HSP’s are so different (= longer HSP distance).
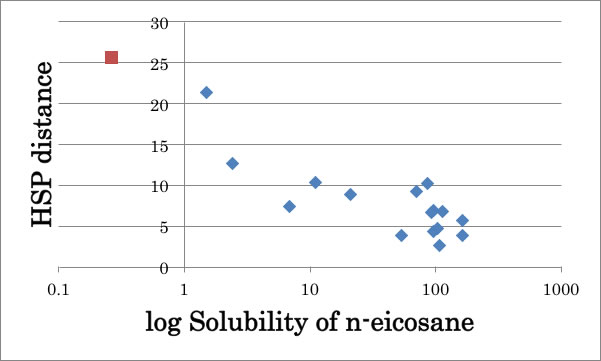
I need not use Methanol cluster model for this case. There are many examples in polymer solubility that methanol play like this.
But when I examine the solubility of Oleic Acid, something strange happen with Alcohol solvents.
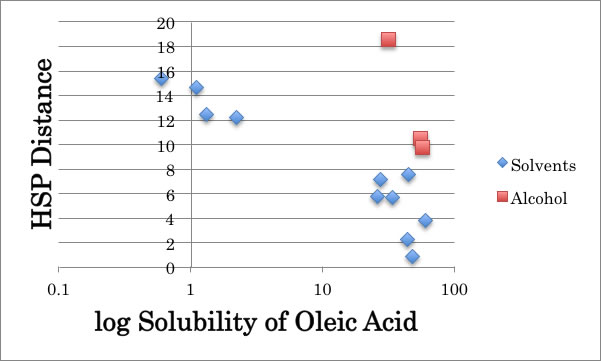
The Methanol dissolves so much amount of Oleic Acid than HSP distance expected. So for this case, we need to introduce “Cluster Model”. The HSPs of Oleic Acid are [16.0, 2.8, 6.2], so methanol should reduce dP and dH values as to form Cluster. When I think of Cluster, there are 2 possibilities. One is methanol’s n-mer cluster, and the other is methanol – solute cluster (several methanol and oleic acid cluster). I can’t distinguish there two, but cluster formation energies are come from hydrogen bonding force, so the aggregation size will be affected by the relationship Methanol HB energy and solute’s HB energy. So the aggregation size is dependent to solute and can’t assign identical numbers.
The shape of cluster:
In the Raymond book, they suggest linear cluster.
When I think of glycol ether, HSPs change become like that.
CH3OCH2CH2OH [16, 8.2, 15]
CH3OCH2CH2OCH2CH2OH [16.2, 7.8, 12.6]
CH3OCH2CH2OCH2CH2OCH2CH2OH [16.2, 7.6, 12.5]
So, if hydroxyl group loose dH energy by forming hydrogen bonding cluster, though it has open hydroxyl group at end, it will not reach Oleic Acid’s HSP=[16.0, 2.8, 6.2].
I thought of 3 dimensional clusters to erase open hydroxyl group. Let’s think of water in oil emulsion with methanol surfactant. The hydroxyl group has large surface energy and aggregate to reduce surface energy in oil. And CH3 group is stick into oil. How many methanol molecules make one cluster? Dimer is too few. Oil can easily access to hydroxyl group. Tri-mer will make plane structure so oil can access from top or bottom direction. Tetra-mer can make pyramid structure so the methyl groups locate every vertex. Every hydroxyl groups are inside the pyramid and form hydrogen-bonding network. Then that pseudo molecule’s surface, it exist only methyl group and from solute position, no oxygen can be seen.
So I assume that HSP are similar to,
CH3-O-t-Bu [14.8, 4.3, 5]
CH3-C(=O)-t-Bu [15.3, 6.3, 3]
CH3-COO-tBu [15, 3.7, 6]
And these HSPs are very similar to Oleic Acid.
There is no exact evidence, but when I divided Methanol dP and dH value with aggregation number 3 or 4, it becomes.
Methanol [14.7, 12.3, 22.3]
3-mer [14.7,12.3/3, 22.3/3]=[14.7, 4.1, 7.4]
4-mer [14.7, 12.3/4, 22.3/4]=[14.7,3.1, 5.6]
The 4-mer is very similar to CH3-O-t-Bu.
So minimum size micro emulsion, most hydrophobic methanol cluster’s HSPs are [14.7, 3.1, 5.6].
Drag=Rotate, Drag+Shift=Larger/Smaller, Drag+Alt or Command(Window key)=Translate.
If you are using HTML5 enable browser such as Chrome, Safari or FireFox (IE9 is out of support), you will see the Canvas with single MeOH and MeOH 4mer with QEQ Charge. Please check the oxygen charge when MeOH make cluster.
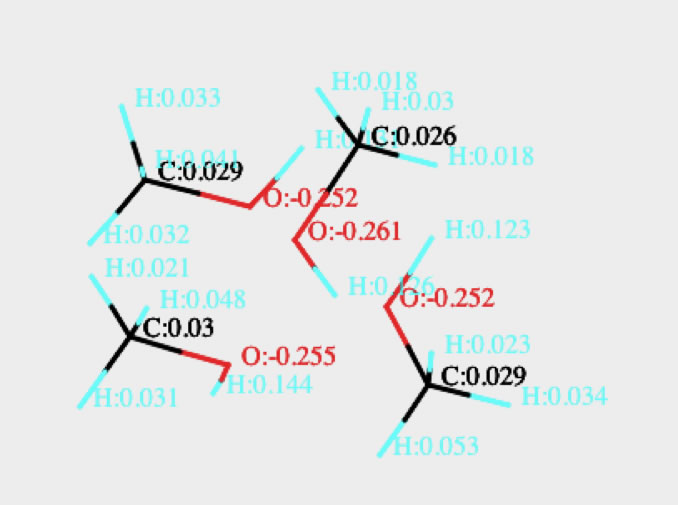
When methanol exists in bulk, it plays as normal alcohol.
Very near to hydrophobic surface like fluorine polymer or inside such polymer, methanol tries to reduce OH group surface energy and form pyramid cluster. And that cluster’s HSP matches to that of polymer‘s and that lead much swelling.
With 4-mer’s HSP, we can easily understand the correlation of HSP distance and solubility of oleic acid.
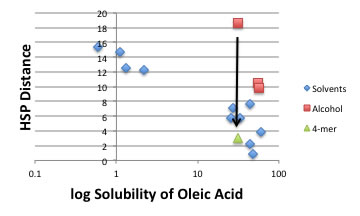
So, bulk methanol [14.7, 12.3, 22.3] to cluster methanol [14.7,3.1, 5.6] HSP will vary according to solute’s hydrogen bonding energy. Other alcohol may make cluster, but pyramid size become larger and can’t stabilize with 4-mer so the cluster formation effect may become small.
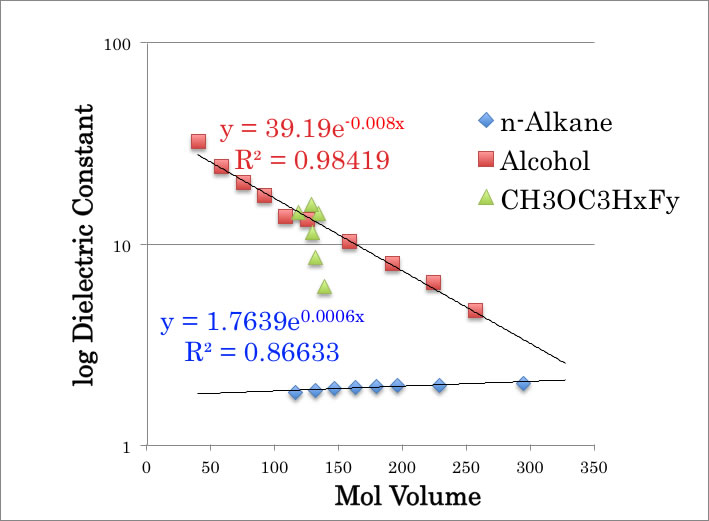
You may feel strange both hydrocarbon polymer and fluorine polymer are hydrophobic though methanol would not make cluster with hydrocarbon polymer. This may come from dielectric constant of compounds. As “Pirika’s rule 3”, molecular volume and log Dielectric constant have good correlation. For n-alkane, Dielectric constant become slightly large according to increase Mol Volume. But 1-substituted of alcohols, Dielectric constant going down as Mol volume going up. The dielectric constant of Perfluorohexane is 1.76, so it is almost same with n-alkane. But CH3OC3HxFy (x+y=7) compounds have very large dielectric constant. And this difference is come from electron negativity of fluorine atom. The fluorine atom charge minus and Hydrogen atom charge plus and it make hydrogen bonding with methanol. Nafion or such ion exchange membrane for fuel cell, they have hydrogen-bonding site from the beginning so methanol will make cluster and crossover via membrane.
Conclusions,
1: It is depend on solute whether Methanol make cluster or not.
2: Even methanol make cluster, the aggregation size is different as solute.
Sorry for this ambiguous conclusion, but if you get strange result of solubility for you solute with methanol, please remember this result.
Please try the number of dP and dH divided by 2, 3, 4. You may get perfect fit.
Please do not stick to the word of 2, 3, 4-mer. I just divided by that number. The important thing is extreme limits.
In my experience, water (methane hydrate case), DMSO, DMF and 1,4-Dioxane also have some strange 3D cluster structure effects.
And sometime it may change diffusion rate in polymer because shape and HSP are changing.
This strange behavior of methanol may cause to cross over of Fuel Cell fluorinated membran. So, close examine of this phenomena is very important.