I received a paper on the Relaxation No. and Hansen Solubility Parameters (HSP) of Magelela’s pulse NMR.
“Fast NMR relaxation, powder wettability and Hansen Solubility Parameter analyses applied to particle dispersibility”というPowder Technology 377 (2021) 545–552
Both Prof. Abbott, who is doing HSP with me, have noted David’s paper.
I have previously blogged about the importance of Electron Donor (ED) and Electron Acceptor (EA) when using Lumisizer for analysis.
For normal (up to fluorine in Periodic Table) acids and bases, we can discuss with δHDo and δHAc.
However, when even d electrons are involved, as in transition metals, ED and EA are also important.
Therefore, I wrote a story about how to perform principal component analysis (PCA) to reduce the 7 dimensions to 3 dimensions, since they cannot be displayed in Hansen space, which can only display up to 3 dimensions.
At that time, the MS Scientific page for the data using Lumisizer was erased due to some problems and cannot be seen now.
So, in this blog, I will use a public paper that links HSP with analysis using pulse NMR as a story and write about the treatment of cases where even d-electrons are involved, such as transition metals.
The hydrogen bonding force of HSP is calculated from the energy of the hydrogen attached to OH or NH, which have high electronegativity, when it forms a bond with an unshared electron pair (lone pair). We decided to further divide the energy into two parts. Brønsted–Lowry’s definition of an acid and a base is simplest: an acid gives a proton and a base receives a proton.
In the HSPiP, since it was based on Abraham’s Acid, Base, it is labeled as δHDo, δHAc (donor/acceptor), but it actually represents an acid and a base.
So, to avoid confusion, I define Lewis’ definition of acid and base as Electron Donor (ED: so-called base) and Electron Acceptor (EA: so-called acid).
This routine to estimate EA,ED is not yet included in ver.5.4 of HSPiP.
However, they are very useful parameters for machine learning (ML) and material informatics (MI).
We will consider how to provide it to HSPiP users slowly from now on.
What does the NMR Relaxation No. mean?
The solvents and Relaxation No. in this paper are as follows. Let’s challenge the researcher who thinks he/she is the one to do it.
Since the Smiles structural formula of the molecule is known, the HSP could easily be calculated using Y-MB.
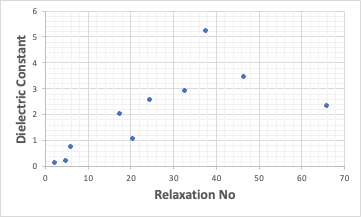
When I first read this paper, I felt this depended on the dielectric constant of the solvent.
When I plotted it, it is roughly correct.
But it deviates greatly where Rno is large.
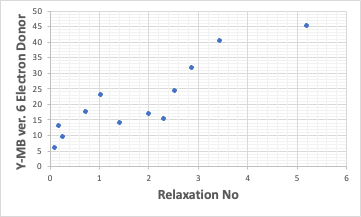
As with the Lumisizer in the results, the Electron Donor correlated best with Relaxation No.
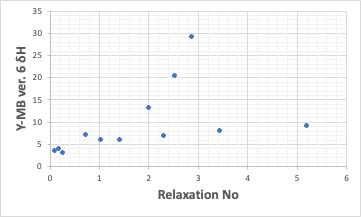
The correlation with δH is as follows. This is not suitable for QSAR.
If the solvent structure is available, it can be calculated by Y-MB. From the ED of the (new) Y-MB output, the Relaxation No. is determined.
In other words, we can calculate solvents from one end to the other, and then patent the solvent with the optimal Relaxation No.
There is a problem, however: in HSP theory, the solvent mixture can be calculated as a volume fraction. However, it is not known what the effect of the solvent mixture would be on something like adsorption on the nanoparticle surface, as in this case.
Is the quickest way to get academic users and some core users to use the new Y-MB?
This inorganic material is silica-coated ZnO. It may be used for cosmetics such as UV absorption. I am still studying in that field and do not know much about it.
If we are dealing with catalysts, ceramics, glass, etc., we can do a lot with this kind of data.
As explained in the ink design for inkjet, it is a matter of HSP matching when dealing with inorganic materials, polymers, and solvents in three systems.
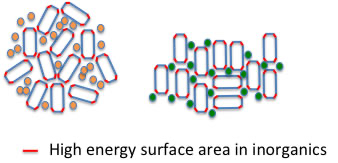
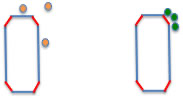
For example, on the inorganic surface of such slurry ink, a high-energy surface is created when the inorganic material is pulverized. At that time, solvents with high ED (green spheres) are adsorbed on the energetic surface. However, solvents with low ED (orange spheres) are not adsorbed, so the particles stick to each other on the energetic surface, creating a bulky structure.
And think about putting polymers in there.
If amide solvents are well adsorbed, polyvinylpyrrolidone (PVP) would be a good match.
For alcohol-based solvents, polyethylene carbonate (PEC) would be a good choice.
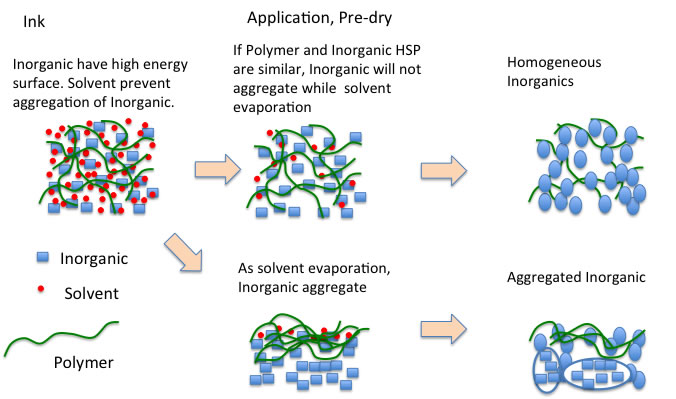
Pre-curing is performed to skip solvent from such ternary systems.
If the functional groups of the polymer can be well coordinated to the surface of the inorganic material after the solvent has flown off, a homogeneously dissolved inorganic material is finally produced.
If the polymer and inorganic material are not well matched during the pre-cure stage, condensation of the inorganic material will occur and that portion will not dissolve well.
I wonder if these “lies” that we have seen will become clearer with pulsed NMR.
I look forward to the progress of this research.
