Trouton’s general rule between latent heat of evaporation and boiling point is known to have the following relationship for nonpolar compounds.
Lb/Tb=21 cal/g-mol
The latent heat of evaporation in this case is the latent heat of evaporation at the boiling point, which can be easily extended to 25 degrees.
As a test, the latent heat of evaporation at 25°C plotted against the boiling points of the compounds is shown below.
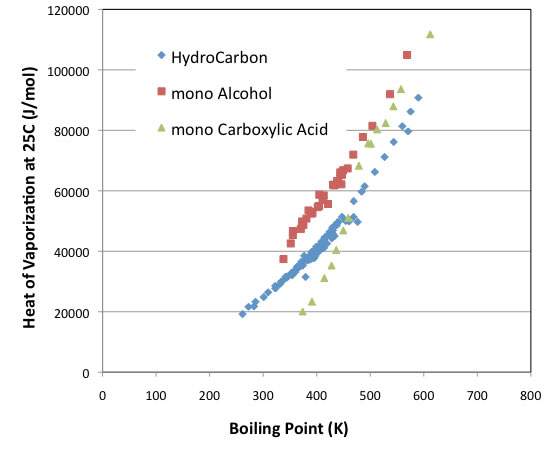
The intercept varies depending on the type of compound, but the curves are usually parallel.
The exceptions are carboxylic acid compounds, which behave similarly to alcohols with boiling points above 480 K. Below that, however, the latent heat of evaporation is even smaller than that of hydrocarbon compounds without functional groups.
The following graphs clearly show why this is the case.
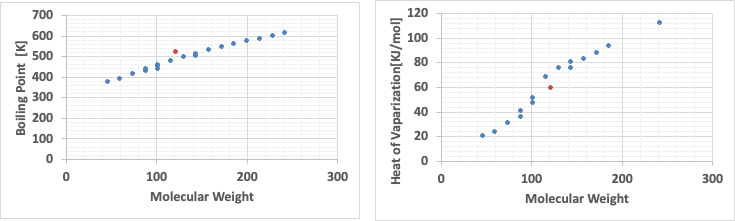
When the boiling point is plotted against molecular weight, there is no particular problem; the boiling point increases as the molecular weight increases.
However, when looking at the latent heat of evaporation, the latent heat of evaporation is small up to a molecular weight of around 100, while above that, the latent heat of evaporation is large.
Therefore, it can be seen that carboxylic acids deviate from trouton’s general rule because the evaporation latent heat of evaporation of low molecular weight carboxylic acids is small.
The orange dot will be the BENZOIC ACID.
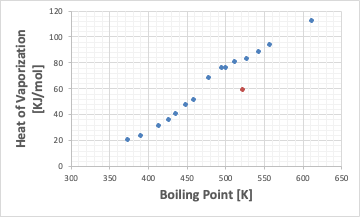
So, when I plot BENZOIC ACID on a graph of boiling point vs. latent heat of evaporation, it comes to a very strange place.
Although it has a high boiling point, the latent heat of evaporation is very low. Therefore, when the vapor is cooled and liquefied, the condensation heat is also small. Moreover, its melting point is as high as 122.4℃, so it solidifies quickly when cooled.
This is the reason why BENZOIC ACID often causes piping blockages during distillation.
This phenomenon is well known in the field of chemical engineering dealing with distillation.
As a test, I made 3D coordinates of a dimer of acetic acid and calculated its charge using the charge equilibrium method (QEQ).
I don’t know why I didn’t do this before, but I had not displayed the dipole moment value, even though I had calculated it internally. The dipole moment of this dimer is 0.409, which is very small.
Drag=Rotate, Drag+Shift key=Zoom in or out, Drag+Command or Alt key=Move.
The monomer results in a large dipole moment.
It is said that IR in the gas phase actually shows that the dimers are still hopping around even in the gas phase.
Essentially, the latent heat of evaporation increases when a 2- or 3-dimensional network of hydrogen bonds is created. Compared to hydrocarbons, the curve for alcohol compounds comes to the top.
However, when closed hydrogen bonds are formed, such as bimolecular hydrogen bonds, the latent heat of evaporation becomes small.
The latent heat of evaporation is an important index used to determine Hansen’s solubility parameter. I always have trouble dealing with carboxylic acids.
