This morning’s newspaper reported that Pfizer’s paxilovid has reduced the severity of the disease by 90% in the final trial. They are also saying that it is effective against the Omicron strain.
This drug, Paxilovid, is a fixed-dose combination of an antiviral drug (PF-07321332) and an anti-HIV drug, Ritonavir.
Hansen solubility parameters for the antiviral drug (PF-07321332) can be found here .
Hansen solubility parameters for ritonavir can be found here, so I’ll summarize the two now.
PF-07321332( Pfizer ):CC(C)(C)C(NC(=O)C(F)(F)F)C(=O)N1CC3C(C1C(=O)NC(C#N)CC2CCNC2=O)C3(C)C
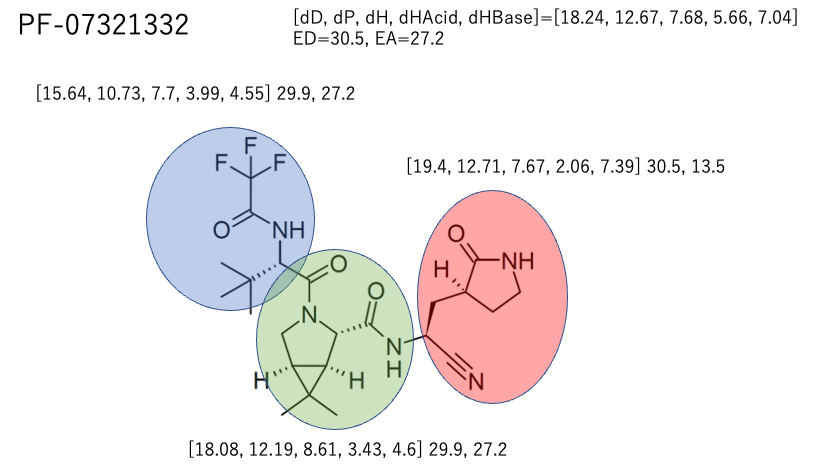
For Pfizer’s PF-07321332, we have explained here that it is better to divide it into three parts and calculate the HSP of each part. This is because if you look at the average value of a large molecule, the solubility of each part will be ambiguous.
Ritonavir was calculated a long time ago, so I discussed it as an average.
This time I’m not sure, so I divided it into three categories with Ayer.
There may be some objections.
At any rate, I am a complete amateur in pharmacology, so if you have an expert opinion, I will follow it unconditionally. Please comment.
Ritonavir(リトナビル): CC(C)c4nc(CN(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OCc3cncs3)cs4
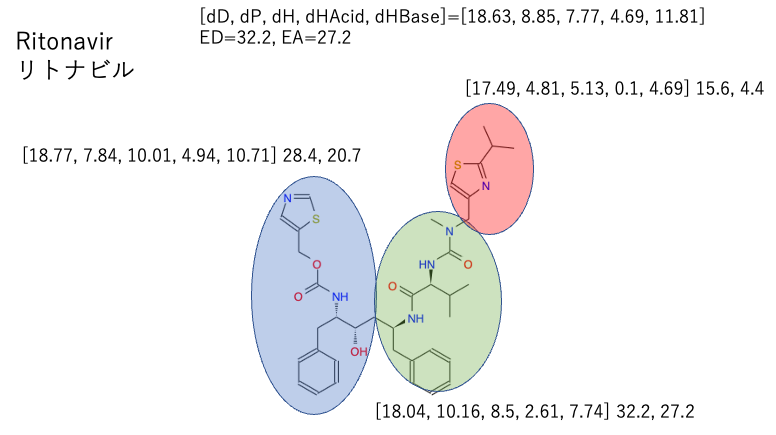
This time, since it is a combination of two ingredients, I tried to find out where the average value of the two drugs and the HSP of each part of the drug are located in the Hansen space.
In the canvas below, you can see many small blue spheres. Each one of them represents a solvent. Click on a sphere. Click on the sphere and it will show you which solvent it is. These 88 solvents are the solvents that Dr. Hansen used in his early dissolution tests. The red sphere is the HSP of each part. The red sphere is the HSP of each part, the average HSP is the red sphere and the large light blue or green sphere of radius 5.
Drag=Rotate, Drag+Shift=Magnify, Drag+Command key or Alt key=Move.
Let’s zoom in by dragging with the mouse for a better view.
The light blue (Ritonavir) and green (PF-07321332) large spheres overlap quite well.
And Rito-right and PF-left are out of the big sphere. If you look at the molecules as an average, you will miss the solubility of this substructure.
Click on the blue spheres to check the HSP of the solvents around the molecule.
Next, let’s look for the Rito-Center and PF-Center inside the big sphere.
We can see that the structure of the middle part is completely different between Ritonavir and PF-07321332, but the HSP is very close. It is expected that the two centers will blend well together.
“This ritonavir, when used in combination with a type of antiviral drug called a protease inhibitor, has the effect of maintaining high blood levels of the protease inhibitor,” from Dr. Kutsuna Kenshi’s blog.
If it’s not working as anti-HIV drug, then to increase the solubility, we need to find the structure which has HSP [dD, dP, dH, dHAcid, dHBase]=[18.08, 12.19, 8.61, 3.43, 4.6] of PF-Center by HSPiP. I think it is possible to use substituent to increase solubility in side chain.
Originally, why am I, a non-pharmacologist, providing the HSP of drugs?
If drugs can penetrate cell membranes, what is the relationship between HSP of drugs and HSP of cell membranes? and
What is a XXX inhibitor, after all?
When a virus replicates itself, it eats food.
Why do they recognize it as food?
Even in humans, taste and smell have a lot to do with HSPs.
Viruses also have this
